
By Daniel Igboekwe
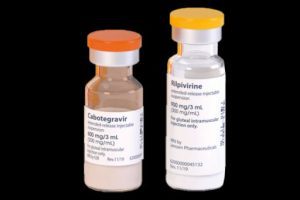
ViiV Healthcare, a company that focuses on HIV treatment and prevention, has announced that it will significantly increase the supply of a long-lasting HIV prevention drug called cabotegravir (known as CAB LA for PrEP) for low- and middle-income countries (L&MICs).
They plan to provide at least two million doses of this injectable medication in 2025 and 2026. This is three times more than what they made available in 2024.
The goal is to meet the growing need for HIV prevention in regions with the highest number of cases and limited healthcare access, such as Sub-Saharan Africa.
Why this matters
HIV is still a big problem worldwide. In 2023 alone, around 1.3 million people became infected. Existing prevention methods, like daily pills, aren’t always practical or accessible for everyone, especially in low-resource settings.
ViiV Healthcare’s long-lasting injectable offers a more convenient option because it doesn’t require daily use, making it easier for people to stay protected.
What is CAB LA for PrEP?
CAB LA (cabotegravir long-acting) is a medication that prevents HIV infection before exposure, a method known as pre-exposure prophylaxis (PrEP). It’s given as an injection that lasts for several months, unlike other PrEP options that need to be taken daily as pills. This long-acting formula is especially helpful for people who might struggle to take daily medication.
ViiV’s approach
ViiV is working quickly to make this injectable option available in the areas that need it most. The U.S. FDA first approved CAB LA for PrEP in 2021 under the brand name “Apretude.” Since then, ViiV has focused on getting approval for the drug in countries with high HIV rates, especially in Sub-Saharan Africa.
Over half of the approvals have been in this region, and nearly 80% have been in low- and middle-income countries.
Efforts to make it affordable
ViiV is offering this drug at a not-for-profit price in low-income countries to make it more accessible. They have already started making it available in countries like Zambia, Malawi, Zimbabwe, Eswatini, and Ukraine. By the end of 2024, they plan to have introduced the drug in 14 countries, most of which are in Sub-Saharan Africa.
Partnership with generic manufacturers
To further reduce costs and increase availability, ViiV is working with the Medicines Patent Pool, an organization that helps make affordable versions of essential medicines.
They signed a licensing agreement in 2022 to allow other companies to produce generic versions of CAB LA. ViiV is helping these companies with technology and expertise to speed up the process.
Why long-acting PrEP is important
Long-acting PrEP like CAB LA is a game-changer for people at high risk of HIV infection, especially in Sub-Saharan Africa, where young women and girls face a high risk.
The injectable option offers a more convenient and discreet way to prevent HIV, which can fit better into people’s lives and increase the chances of staying protected.
In summary, ViiV Healthcare is ramping up its supply of an important HIV prevention drug to help reduce infections in the places that need it most, with a focus on affordability and accessibility.




Leave a Reply
View Comments